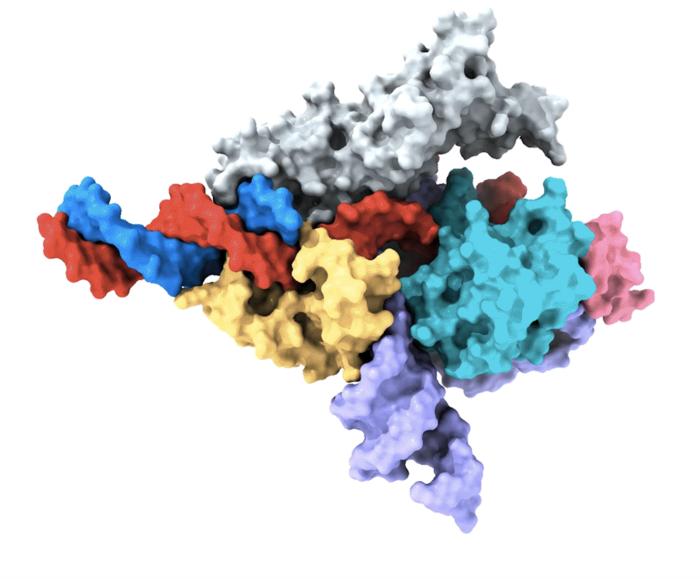
Recently David Liu announced that Prime Medicine will likely submit the first human trial application in 2024. The standard version of CRISPR uses an RNA guide to find the editing site in the genome. Prime editing, on the other hand, also uses the same RNA molecule to direct the correction, in short, to specify what to do as well as where to go.
This insight blossomed in Andrew Anzalone’s mind a few years ago during his PhD at Columbia University. The first practical demonstration came with a paper published in Nature in 2019 after joining the Liu’s Lab at the Broad Institute. Since then, this platform has been used in hundreds of experiments to fix all kinds of mutations in vitro and in animal models.
Meanwhile, the company co-founded by Anzalone and Liu has begun work on 18 treatments, the most advanced for chronic granulomatous disease. To learn more, from the eureka moment to the latest developments, we suggest listening to the Close to the Edge podcast and reading Alex Philippidis’ article in GEN.