Imagine throwing a trillion darts and having every single one hit the bullseye. Achieving this level of precision in gene editing would require highly intelligent delivery of the molecular machinery for DNA repair (CRISPR or one of its variants) into patients’ bodies, reaching only defective cells while bypassing healthy tissues. The benefits would be substantial: maximum therapeutic efficiency, zero waste, and reduced risks in terms of toxicity, immunogenicity, and unwanted mutations. How can such precise targeting be achieved? By acting on multiple levels, explain Jennifer Doudna and three researchers from her Innovative Genomics Institute. See their review article, Targeted delivery of genome editors in vivo in Nature Biotechnology.

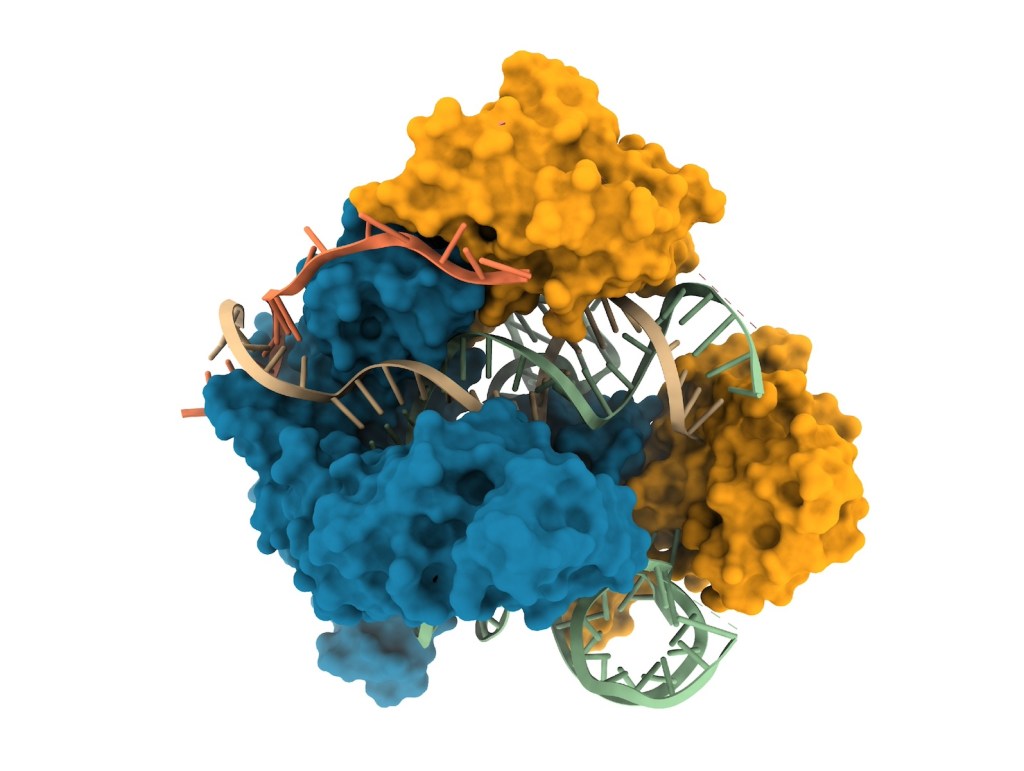



![nanoparticle MIT[1423]](https://mycrispr.blog/wp-content/uploads/2017/11/nanoparticle-mit1423.jpg) Suppose you have developed the winning weapon to defeat certain genetic diseases by reliably correcting pathogenic mutations. There is still a problem: how do you march onto the battlefield, inside sick cells? The weapon is the genome-editing machinery, and the most efficient vessel ever tested are lipid nanoparticles. With this approach, described in a study published in
Suppose you have developed the winning weapon to defeat certain genetic diseases by reliably correcting pathogenic mutations. There is still a problem: how do you march onto the battlefield, inside sick cells? The weapon is the genome-editing machinery, and the most efficient vessel ever tested are lipid nanoparticles. With this approach, described in a study published in