
After the uproar over the de-extinction of dire wolves, bioethicist Arthur Caplan asked in Plos Biology: “Should scientists be allowed to bring distant human ancestors back to life?” The Italian edition of Scientific American invited me to investigate how technically difficult it would be to de-extinct a Neanderthal and what the risks and benefits of such a project might be. Below are the statements provided by the specialists I consulted.
Continue reading



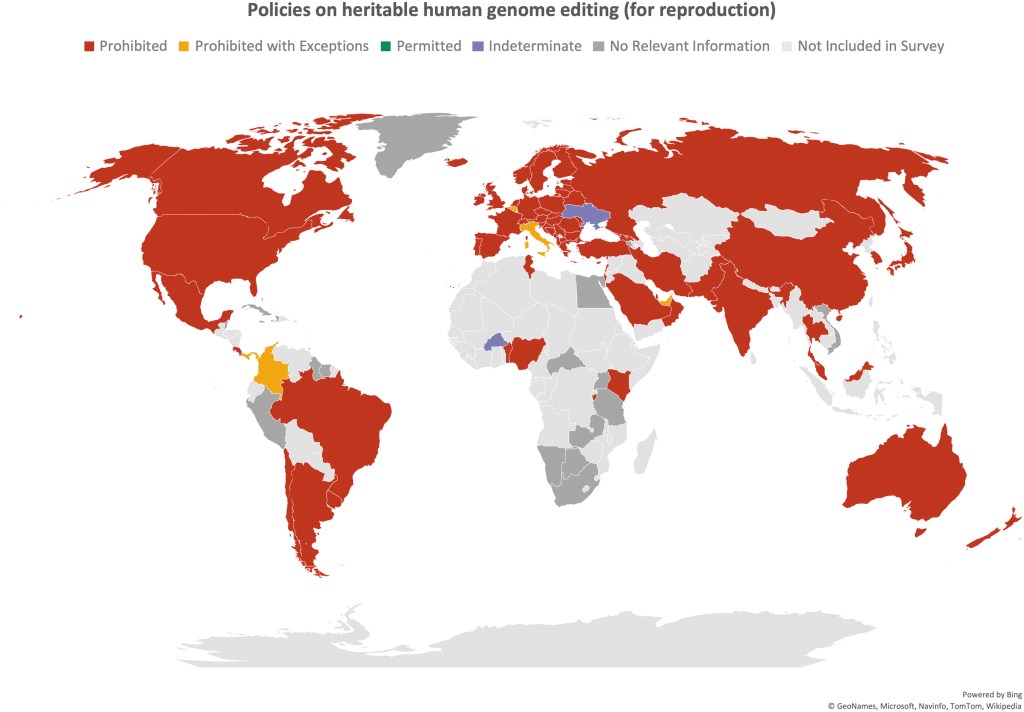
 Where is Jennifer Doudna? This is the first thought most journalists had – me included – when reading the list of signatories to the call for the moratorium on heritable genome editing just published by
Where is Jennifer Doudna? This is the first thought most journalists had – me included – when reading the list of signatories to the call for the moratorium on heritable genome editing just published by