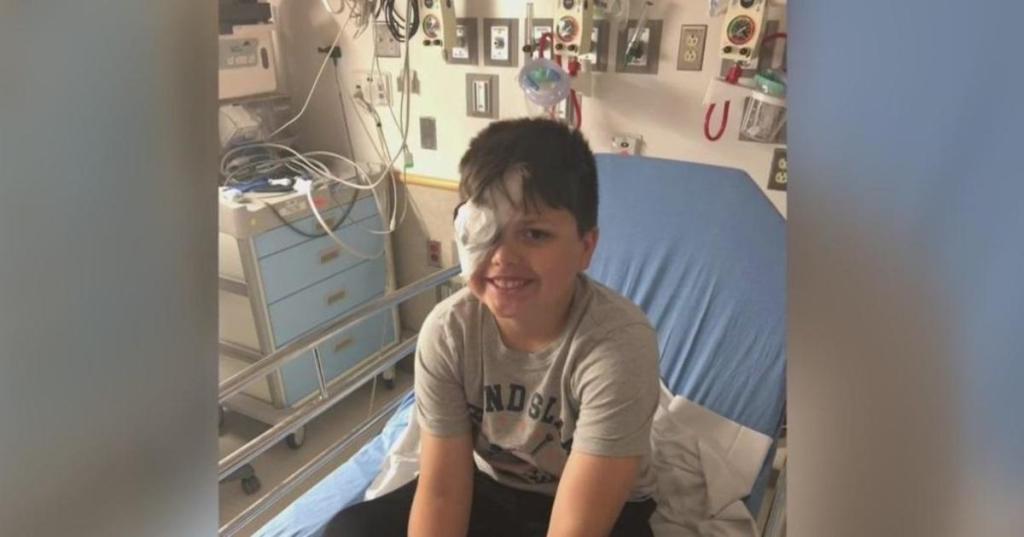
This photo shows the first American “non-experimental” patient leaving the hospital after completing the CRISPR-based treatment for sickle cell anemia (Casgevy). The New York Times detailed this “official first,” which followed the success of a clinical trial involving dozens of patients like Victoria Gray. We still know little about the first person who is beginning treatment in Europe since this therapy became an “approved drug”. According to Osservatorio Terapie Avanzate he is a young adult (23 years), who arrived in Italy in 2014 and living in the Umbria region, where is being also treated. Undergoing cell extraction and reinfusion of edited cells is an invasive and exhausting process, but now the American Kendric Cromer (12 years old) and other “first patients” can hope to lead full lives—without painful crises or blood transfusions. Best of luck!