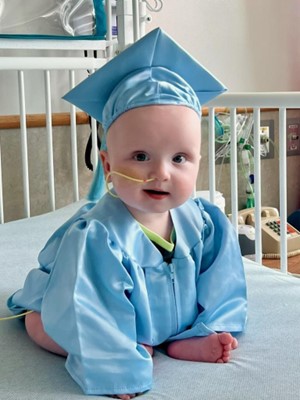
The coordinated effort that last spring saved the life of little KJ Muldoon earned widespread and enthusiastic media coverage. But between the invention of the treatment and its delivery to the patient lay a lesser-told story: an unprecedented manufacturing sprint. Genetic Engineering & Biotechnology News organized an online roundtable led by its deputy editor in chief, Julianna LeMieux, to discuss how therapeutic components were produced quickly, cost-effectively, and to clinical-grade standards.
Continue reading