New Approach Methodologies (NAMs) have a bright future ahead, but they should be seen as complementary rather than alternative to classical experimentation.
Regulatory and funding agencies in the U.S. and Europe are promoting ambitious initiatives to foster the development and adoption of advanced systems capable of testing the effects of drugs and other substances without using animal models. The hope is that biomedical research can become more ethical, safer, and cheaper. But the challenge is complex, and the requirements vary depending on the application. As a result, some voices urge a faster “transition,” while others warn that rushing the process could be risky. Recently published articles in leading scientific journals capture this polarized debate, but they also hint at a possible middle ground.
“Alternatives to animal testing are the future—it’s time that journals, funders and scientists embrace them” reads a commentary published at the end of October in Nature, written by Todd J. Herron and a group of U.S. and U.K. researchers. “Why simply ending animal testing isn’t the answer in biomedical research” is the second piece, appearing in the same issue and authored by American scientist K. C. Kent Lloyd. To navigate between these contrasting positions, it may be useful to start from the feature published this summer by Sara Reardon in Science.
We are living through an extraordinary period of progress in experimental approaches within the life sciences. On one hand, genetic editing is refining animal models, for example, by developing mouse lines with mutations mimicking the progression of human diseases, or by fixing pathogenic mutations to prove the feasibility of gene-editing therapies. Take the case of progeria. In the videos accompanying an encouraging Nature paper from 2021, the sick mouse struggles, while the gene-edited ones appear full of energy, offering hope to patients and their families.
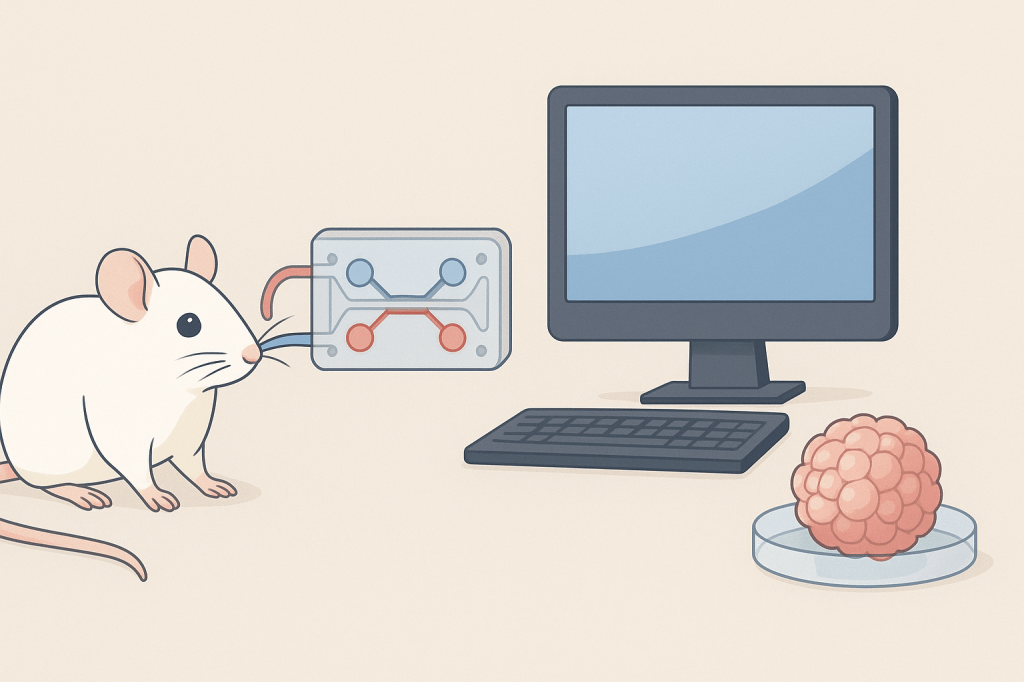
On the other hand, NAMs—an acronym that stands for New Alternative Methods or New Approach Methodologies—are advancing rapidly, and they come in at least three types.
First, there are in vitro NAMs, such as 3D organ models (so-called organoids), which make it possible to gather valuable information without sacrificing sentient creatures. This category also includes organs-on-chips—small microfluidic devices equipped with channels hosting layers of cells and allowing controlled flows of nutrients and waste.
Then there are in silico NAMs, computational approaches that predict how biological systems respond to drugs or other perturbations, a field booming thanks to the promises of artificial intelligence. Finally, in chemico techniques assess molecular interactions outside of cells and organisms.
To properly frame these developments, it’s important to remember that animal experimentation already follows the “three Rs” principle: Replace, Reduce, Refine. This means aiming to replace animal models whenever scientifically possible, minimize the number of animals used, and refine procedures to reduce suffering. The latest data published in Italy confirms a decline in the number of animals used for scientific research (365,130 in 2023 compared to 548,933 in 2019). In the United States, a scientific superpower, tens of millions of laboratory animals are used each year to test food, drugs, and chemicals, as well as for basic research. Animal rights advocates (and many scientists) hope that next-generation approaches will give a strong boost to the Replace component of the three Rs. International policies are moving in the same direction, though with some stops and starts.
In spring 2025, the U.S. Food and Drug Administration (FDA) and the Environmental Protection Agency (EPA) announced plans to encourage (but not require) the adoption of NAMs in an increasing range of fields. The FDA, in particular, launched a pilot program allowing certain companies to use next-generation approaches to select new monoclonal antibodies for clinical trials. Meanwhile, the share of NIH-funded projects using non-animal tests has risen, from just 1% in 2000 to 8% in 2024. The National Institutes of Health also announced the creation of the Office of Research Innovation, Validation, and Application (ORIVA), which will increase funding for non-animal approaches, promote training in the field, and raise awareness of their value.
According to Science, Europe is also moving: next year, the European Commission plans to publish a roadmap to phase out animal testing for chemical substances, while the European Medicines Agency (EMA) is evaluating how to handle NAM-generated data in clinical trial applications.
However, Herron and colleagues lament in Nature that the scientific community is reluctant to abandon old methods: they note, for instance, that researchers using NAMs find it harder to publish in high-impact journals or to convince funding reviewers. Lloyd, on the other hand, highlights the current limits of NAMs: while they are already useful for fields like toxicology, none of these approaches can yet reveal how a specific gene works in a person, or how disease progression affects an entire organism.
Both commentaries cite the same fact: 86% of drug candidates fail in human trials. Herron and colleagues see this as evidence of the limits of animal models and call for broader use of advanced human-based approaches. Lloyd, however, argues that the 86% failure rate may also reflect other factors (such as flawed experimental design) that could persist even in NAM-based studies.
Overall, everyone recognizes the great potential of new technologies, so it would be a mistake to portray the debate as a clash between supporters and detractors. No researcher is happy about sacrificing mice and other animals for biomedical progress, and the new methodologies are widely welcomed, as long as they are viewed as complementary rather than alternative to current techniques, especially for delicate and complex applications. It would be unfortunate if the premature adoption of insufficiently validated technologies led to accidents: history shows that such events can stall progress for years, delaying the very transitions they were meant to accelerate.
(translated from Osservatorio Terapie Avanzate)
