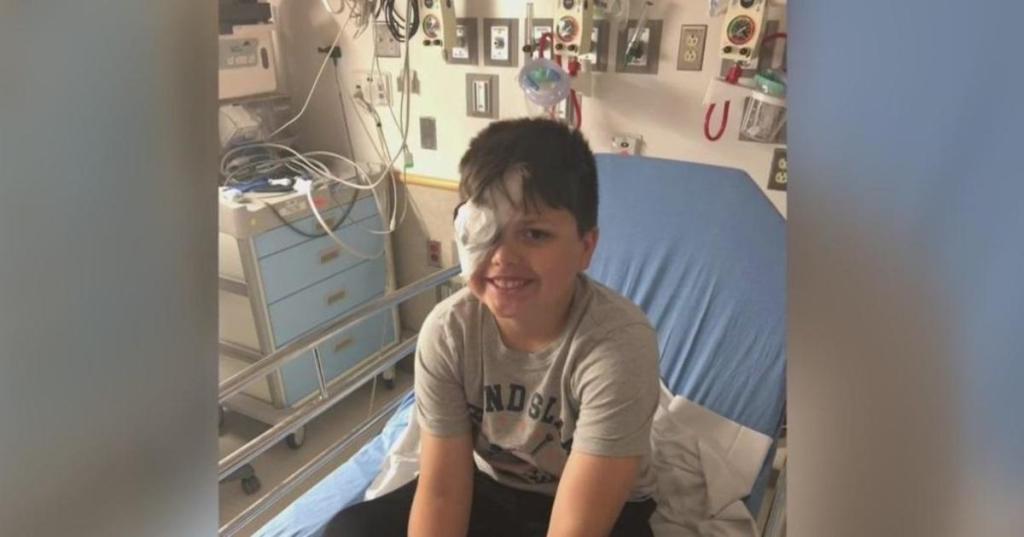
Jacob Peckham, 11, can see much better after receiving an experimental CRISPR-based treatment. The American child, a carrier of a genetic defect that impairs the retina, has had surgery on only one eye and hopes to complete the treatment in the future. However, his wish is unlikely to be granted because the company that developed the treatment (Editas) had to abandon the program due to affordability issues. To give a future to treatments for rare diseases such as this one, insists editing pioneer Fyodor Urnov, it is crucial to build a new model for research, development, and production – that is, to simplify, standardize, integrate, scale up.
